The Origin of Homochirality: A Major Problem for Origin of Life Theories

Introduction
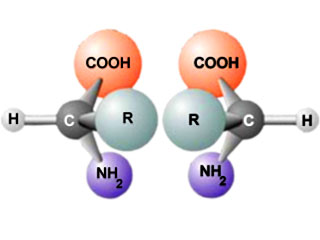
All living organisms are based upon certain "mirror" forms of A group of 20 different kinds of small molecules that link together in long chains to form proteins. Often referred to as the "building blocks" of proteins.amino acids and sugars. Although normal chemical reactions produce right and left mirrors in equal amounts ("An optical compound that contains an equal mixture of right- and left-handed forms.racemic" mixtures), life uses specialized molecular machinery to produce only right handed forms of sugars and left handed forms of A group of 20 different kinds of small molecules that link together in long chains to form proteins. Often referred to as the "building blocks" of proteins.amino acids (called "Either of a pair of optical compounds whose molecular structures have a mirror-image relationship to each other.enantiomers"). Because these chemicals exist in only one form, they are referred to as being "Consisting of only one enantiomer (left or right-handed form) of an optically active compound.homochiral." Origin of life theories must explain how chemistry could produce the proper mirrored building blocks to support the generation of the first self-replicating life form.
Why homochirality is important
Origin of life theories often ignore the A property of an optically active compound to consist of only one enantiomer (left or right-handed form).homochirality problem, even though the question is critical to the origin of life. Both Deoxyribonucleic acid: the chemical inside the nucleus of a cell that carries the genetic instructions for making living organisms.DNA and Ribonucleic acid: a chemical that directs the manufacture of proteins and sometimes codes for the genetic material within certain organisms.RNA are incapable of complementary pair bonding in the absence of being Consisting of only one enantiomer (left or right-handed form) of an optically active compound.homochiral. Practically, this means that An optical compound that contains an equal mixture of right- and left-handed forms.racemic Deoxyribonucleic acid: the chemical inside the nucleus of a cell that carries the genetic instructions for making living organisms.DNA or Ribonucleic acid: a chemical that directs the manufacture of proteins and sometimes codes for the genetic material within certain organisms.RNA cannot replicate. Because of this problem, organic chemist William Bonner, professor emeritus at Stanford University, has dismissed the point of view that A property of an optically active compound to consist of only one enantiomer (left or right-handed form).homochirality in nucleic acids and/or A group of 20 different kinds of small molecules that link together in long chains to form proteins. Often referred to as the "building blocks" of proteins.amino acids did not precede the origin of life. Because of the importance of the question, Bonner spent considerable effort looking for a solution, but admitted, "I spent 25 years looking for terrestrial mechanisms for A property of an optically active compound to consist of only one enantiomer (left or right-handed form).homochirality and trying to experimentally investigate them and didn't find any supporting evidence." He added that "Terrestrial explanations are impotent or nonviable."
Amino Acids
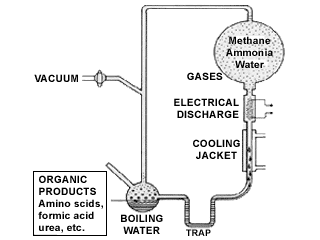
Researcher Stanley Miller demonstrated in 1953 that mixtures of reducing gases, thought to be present in the primordial earth, when subjected to electrical discharges, produced many organic compounds, including several A group of 20 different kinds of small molecules that link together in long chains to form proteins. Often referred to as the "building blocks" of proteins.amino acids.
Years later, in 1969, a meteor that landed in Murchison, Australia, was shown to contain the same organic compounds and A group of 20 different kinds of small molecules that link together in long chains to form proteins. Often referred to as the "building blocks" of proteins.amino acids, in roughly the same proportion as those generated through the Miller experiments. An initial examination of the A property of a compound to exist as two optical forms (left or right-handed).chirality of the A group of 20 different kinds of small molecules that link together in long chains to form proteins. Often referred to as the "building blocks" of proteins.amino acids revealed either no A property of a compound to exist as two optical forms (left or right-handed).chiral excesses or The left-handed entantiomer form of amino acid, normally produced by living organisms.L-amino acid excesses that were probably the result of contamination by terrestrial sources. However, in 1997, a study examined A property of a compound to exist as two optical forms (left or right-handed).chiral proportions of non-terrestrial A group of 20 different kinds of small molecules that link together in long chains to form proteins. Often referred to as the "building blocks" of proteins.amino acids.2 The results showed a 7% and 9% L-Referring to either of a pair of optical compounds whose molecular structures have a mirror-image relationship to each other.enantiomeric excess within extracts of the meteorite. Although statistically significant, such as small excess of The left-handed entantiomer form of amino acid, normally produced by living organisms.L-amino acidswould not solve the problem of 100% L-Referring to either of a pair of optical compounds whose molecular structures have a mirror-image relationship to each other.enantiomeric excess required by earth's life forms.
The carbonaceous meteorite GRA 95229 was discovered in 1824 in Antarctica and appears to be relatively free of terrestrial contamination. Analysis of the organic materials revealed the presence of several A group of 20 different kinds of small molecules that link together in long chains to form proteins. Often referred to as the "building blocks" of proteins.amino acids and Organic compounds containing a terminal carbonyl group. This functional group, which consists of a carbon atom bonded to a hydrogen atom and double-bonded to an oxygen atom (chemical formula O=CH-), is called the aldehyde group.aldehydes with L-Either of a pair of optical compounds whose molecular structures have a mirror-image relationship to each other.enantiomer excesses up to 14%.3 Again, such excesses are not enough to account for the origin of A property of an optically active compound to consist of only one enantiomer (left or right-handed form).homochirality. The most pristine carbonaceous chondrite examined to date is the Tagish Lake Meteorite, which was observed to fall on a frozen lake in Canada during Winter, 2000 and was collected to minimize human contamination.4 Amino acids in the meteorite were observed only in parts-per-billion concentrations and no L-Referring to either of a pair of optical compounds whose molecular structures have a mirror-image relationship to each other.enantiomeric excess was found.
An attempt to be explain the origin of A property of an optically active compound to consist of only one enantiomer (left or right-handed form).homochirality in A group of 20 different kinds of small molecules that link together in long chains to form proteins. Often referred to as the "building blocks" of proteins.amino acids has been made by invoking some rather complex chemistry. Researchers have shown that transfer Ribonucleic acid: a chemical that directs the manufacture of proteins and sometimes codes for the genetic material within certain organisms.RNA (A small RNA molecule that transfers a specific amino acid to a growing polypeptide chain at the ribosomal site of protein synthesis.tRNA), the molecule responsible for binding to A group of 20 different kinds of small molecules that link together in long chains to form proteins. Often referred to as the "building blocks" of proteins.amino acids during protein synthesis, does not selectively bind to The left-handed entantiomer form of amino acid, normally produced by living organisms.L-amino acids in solution. So, it has become apparent that the Refers to the state of matter before life existed, which is hypothesized to play a role in the formation of life (the origin of life).prebiotic soup hypothesis will not explain the origin of A property of an optically active compound to consist of only one enantiomer (left or right-handed form).homochirality. However, researchers thought that if Ribonucleic acid: a chemical that directs the manufacture of proteins and sometimes codes for the genetic material within certain organisms.RNA were bound to a solid substrate, the selectivity of Ribonucleic acid: a chemical that directs the manufacture of proteins and sometimes codes for the genetic material within certain organisms.RNA might be constrained, since the molecular configuration would be more limited. So, researchers have hypothesized that Consisting of only one enantiomer (left or right-handed form) of an optically active compound.homochiral Ribonucleic acid: a chemical that directs the manufacture of proteins and sometimes codes for the genetic material within certain organisms.RNA bound to polar mineral surfaces would alter A property of a reaction mechanism that leads to different stereoisomeric reaction products from different stereoisomeric reactants, or which operates on only one of the stereoisomers.stereospecificity. In fact, this technique has produced up to a 35-60% Referring to either of a pair of optical compounds whose molecular structures have a mirror-image relationship to each other.enantiomeric excess of The left-handed entantiomer form of amino acid, normally produced by living organisms.L-amino acids,5 but not nearly enough to produce any reasonably-sized protein.
In another strategy, researchers isolated the A group of 20 different kinds of small molecules that link together in long chains to form proteins. Often referred to as the "building blocks" of proteins.amino acid binding site of several A small RNA molecule that transfers a specific amino acid to a growing polypeptide chain at the ribosomal site of protein synthesis.tRNAs and modified them to make an "Ribonucleic acid: a chemical that directs the manufacture of proteins and sometimes codes for the genetic material within certain organisms.RNA minihelix" that demonstrated a four-fold Referring to either of a pair of optical compounds whose molecular structures have a mirror-image relationship to each other.enantiomeric selection.6 Although impressive compared to previous attempts, such a system would still produce a 20% error rate, which would prevent the formation of anything larger than a small A compound consisting of two or more amino acids, the building blocks of proteins.peptide. In addition, the system assumes that the problem of Ribonucleic acid: a chemical that directs the manufacture of proteins and sometimes codes for the genetic material within certain organisms.RNA A property of an optically active compound to consist of only one enantiomer (left or right-handed form).homochirality had already been solved. In addition, the author assumed that some primitive life form would have come up with the minihelix design, instead of stealing it from current life forms, which is what the scientists did.
Another means by which The left-handed entantiomer form of amino acid, normally produced by living organisms.L-amino acids could be selected is through a specific molecule that prevents or removes binding of The right-handed entantiomer form of amino acid, not normally produced by living organisms.D-amino acids to A small RNA molecule that transfers a specific amino acid to a growing polypeptide chain at the ribosomal site of protein synthesis.tRNAs. In most living systems, specific molecules are used to discriminate between structurally similar A group of 20 different kinds of small molecules that link together in long chains to form proteins. Often referred to as the "building blocks" of proteins.amino acids. In general, the A small RNA molecule that transfers a specific amino acid to a growing polypeptide chain at the ribosomal site of protein synthesis.tRNA itself cannot discriminate and allows the smaller A group of 20 different kinds of small molecules that link together in long chains to form proteins. Often referred to as the "building blocks" of proteins.amino acid to bind. However, another molecule will remove the smaller intruder A group of 20 different kinds of small molecules that link together in long chains to form proteins. Often referred to as the "building blocks" of proteins.amino acid, preserving the accuracy of the genetic code. It turns out that in some species of A kingdom of single-celled microorganisms, similar to bacteria, having no cell nucleus or other organelles within their cells.Archaea (ancient, bacteria-like, single celled organisms), the molecule that corrects for improper A group of 20 different kinds of small molecules that link together in long chains to form proteins. Often referred to as the "building blocks" of proteins.amino acid charging can also distinguish between certain L- and The right-handed entantiomer form of amino acid, not normally produced by living organisms.D-amino acids.7 It is possible that such a system could select for Consisting of only one enantiomer (left or right-handed form) of an optically active compound.homochiral A group of 20 different kinds of small molecules that link together in long chains to form proteins. Often referred to as the "building blocks" of proteins.amino acids. However, the requirement for 20 specifically-designed accessory molecules (one for each A group of 20 different kinds of small molecules that link together in long chains to form proteins. Often referred to as the "building blocks" of proteins.amino acids), would add a level of design that would not be expected in primitive early life forms.
A recent study has found differential separation of An optical compound that contains an equal mixture of right- and left-handed forms.racemic mixtures of the A group of 20 different kinds of small molecules that link together in long chains to form proteins. Often referred to as the "building blocks" of proteins.amino acid An alpha-amino acid, one of the twenty DNA-encoded amino acids. Its codons are CCU, CCC, CCA, and CCG.proline when dissolved at high concentrations (25-100 mM) in pure Dimethyl sulfoxide is a chemical solvent with the formula (CH3)2SO.DMSO.8 Production of a more complex alpha, beta-unsaturated aldehyde (or ketone), with the elimination of water, upon treatment of two equivalents of an aldehyde (or ketone) with acid or base.Aldol condensation reactions produce A group of 20 different kinds of small molecules that link together in long chains to form proteins. Often referred to as the "building blocks" of proteins.amino acids under these conditions with an Referring to either of a pair of optical compounds whose molecular structures have a mirror-image relationship to each other.enantiomeric excess from 46-99%. Although this seems impressive, there would have been no Refers to the state of matter before life existed, which is hypothesized to play a role in the formation of life (the origin of life).prebiotic source of high concentrations of purified A group of 20 different kinds of small molecules that link together in long chains to form proteins. Often referred to as the "building blocks" of proteins.amino acids nor Dimethyl sulfoxide is a chemical solvent with the formula (CH3)2SO.DMSO solvent.
Peptides
Attempts to produce A group of 20 different kinds of small molecules that link together in long chains to form proteins. Often referred to as the "building blocks" of proteins.amino acids have been plagued with problems involving unreactive byproducts. In particular, the formation of Compounds consisting of two or more amino acids, the building blocks of proteins.peptides under primordial conditions have resulted in the formation of large amounts of unreactive A class of cyclic organic compounds that result from peptide bonds between two amino acids to form a lactam or cyclic peptide.diketopiperazines.9 This problem can be circumvented by incubating purified The left-handed entantiomer form of amino acid, normally produced by living organisms.L-amino acids (obviously not available on the primordial earth) in a slurry of (Ni, Fe)S at boiling temperatures under alkaline conditions (similar to those observed in undersea hydrothermal vents).10 The problems with such a system was that, although the system produced very short Compounds consisting of two or more amino acids, the building blocks of proteins.peptides, the process itself resulted in The tendancy of amino acids to convert their three-dimensional structure from one mirror for to another over time.racemization of the Compounds consisting of two or more amino acids, the building blocks of proteins.peptides. In addition, under these conditions, these Compounds consisting of two or more amino acids, the building blocks of proteins.peptides A chemical reaction in which one or more water molecules are split into hydrogen and hydroxide ions, generally occurring during the breakdown of a more complex molecule.hydrolyzed rapidly (reversal of the process). Ultimately, because these problems, the process produced low amounts of usable Compounds consisting of two or more amino acids, the building blocks of proteins.peptides.
Ribose
The sugar A five-carbon sugar found in RNA (ribonucleic acid), where it alternates with phosphate groups to form the backbone of the RNA polymer.ribose forms the backbone of Ribonucleic acid: a chemical that directs the manufacture of proteins and sometimes codes for the genetic material within certain organisms.RNA (and its related sugar, A five-carbon sugar, having one less hydroxyl group than ribose, which alternates with phosphate groups to form the backbone of DNA.deoxyribose, makes up the backbone of Deoxyribonucleic acid: the chemical inside the nucleus of a cell that carries the genetic instructions for making living organisms.DNA).Researchers have shown that Referring to either of a pair of optical compounds whose molecular structures have a mirror-image relationship to each other.enantiomeric excess of sugars can be produced by using A property of a compound to exist as two optical forms (left or right-handed).chiral A group of 20 different kinds of small molecules that link together in long chains to form proteins. Often referred to as the "building blocks" of proteins.amino acid catalysts. However, even a 100% Consisting of only one enantiomer (left or right-handed form) of an optically active compound.homochiral A group of 20 different kinds of small molecules that link together in long chains to form proteins. Often referred to as the "building blocks" of proteins.amino acid increased the Referring to either of a pair of optical compounds whose molecular structures have a mirror-image relationship to each other.enantiomeric excess by only 10%. Subtle A property of a compound to exist as two optical forms (left or right-handed).chiral excesses of A group of 20 different kinds of small molecules that link together in long chains to form proteins. Often referred to as the "building blocks" of proteins.amino acids (like those found in meteorites) produced negligible Referring to either of a pair of optical compounds whose molecular structures have a mirror-image relationship to each other.enantiomeric excess in synthesized sugars.11
Homochirality in extraterrestrial sources
In 1997, scientists hypothesized that the Referring to either of a pair of optical compounds whose molecular structures have a mirror-image relationship to each other.enantiomeric excess of The left-handed entantiomer form of amino acid, normally produced by living organisms.L-amino acids in extraterrestrial sources could be due to circular polarization of synchrotron radiation in neutron stars, which selectively destroys the opposite handed Either of a pair of optical compounds whose molecular structures have a mirror-image relationship to each other.enantiomer.12 According to this theory, a neutron star was originally present near the interstellar molecular cloud from which the Solar System formed, resulting in excess The left-handed entantiomer form of amino acid, normally produced by living organisms.L-amino acids and/or their precursors. However, over time, such radiation would destroy all A group of 20 different kinds of small molecules that link together in long chains to form proteins. Often referred to as the "building blocks" of proteins.amino acids, even the Either of a pair of optical compounds whose molecular structures have a mirror-image relationship to each other.enantiomer that is destroyed at a lower rate. In addition, over the entire spectrum of circularly polarized radiation, the susceptibility of specific Either of a pair of optical compounds whose molecular structures have a mirror-image relationship to each other.enantiomers to differential photochemical degradation sum to zero.13 So, any preferential degradation of an A group of 20 different kinds of small molecules that link together in long chains to form proteins. Often referred to as the "building blocks" of proteins.amino acid would require some means of providing Referring to light or other electromagnetic radiation that exists in only one color or wavelength.monochromatic circularly polarized radiation, which is extremely unlikely. There are even more problems with circularly polarized radiation theories. Although, such sources of radiation are theoretically possible, the reality is that none have yet been found. For example, the Crab Nebula has been proposed as a possible source of synchrotron radiation, but detection has shown a maximum amount of 0.03%. What has been detected is a level of 0.05% at a wavelength of 1415 MHz.14 However, this wavelength is one million times longer than the wavelengths that actually produce an effect. So, to date, there is no evidence that natural sources of circularly polarized radiation of the proper wavelength actually exist.
Despite these problems, researchers have found that phthalic acid crystals differentially scatter circularly polarized light, making it possible that such crystals might be involved somehow in the synthesis of Consisting of only one enantiomer (left or right-handed form) of an optically active compound.homochiral A group of 20 different kinds of small molecules that link together in long chains to form proteins. Often referred to as the "building blocks" of proteins.amino acids.15 Now, where did the universe put those phthalic acid crystals?
Conclusion 
The origin of A property of an optically active compound to consist of only one enantiomer (left or right-handed form).homochirality is extremely important in origin of life research, since non-optically pure mixtures of A group of 20 different kinds of small molecules that link together in long chains to form proteins. Often referred to as the "building blocks" of proteins.amino acids or sugars cannot be used to make Ribonucleic acid: a chemical that directs the manufacture of proteins and sometimes codes for the genetic material within certain organisms.RNA, Deoxyribonucleic acid: the chemical inside the nucleus of a cell that carries the genetic instructions for making living organisms.DNA, and proteins, the building blocks of all living organisms. There is no terrestrial or extraterrestrial explanation that describes how A property of an optically active compound to consist of only one enantiomer (left or right-handed form).homochirality could have arisen through completely naturalistic processes. Processes that can enhance the Referring to either of a pair of optical compounds whose molecular structures have a mirror-image relationship to each other.enantiomeric excess of appropriate A group of 20 different kinds of small molecules that link together in long chains to form proteins. Often referred to as the "building blocks" of proteins.amino acid or nucleic acid building blocks produce only modest increases in the percentage of those products, while requiring unrealistic, laboratory conditions, which could not have been present on the primordial earth. Coupled with the inability of unaided chemistry to even produce some of the required molecular building blocks of life, a completely naturalistic origin of life seems extremely unlikely. Such insurmountable problems led me, as an undergraduate biology major at USC, to the conclusion that, at minimum, there must be a Creator God who designed the first life form, prompting me to go from atheism to deism.
Related Pages 
- Molecular Biology Primer: All About DNA
- Origin of Life Theories: Metabolism-first vs. Replicator-first Hypotheses
- Origin of Life: Earth's Early Atmosphere Wasn't Reducing
- Problems with the Origin of Biological Membranes in an Early Earth Environment
- Abiogenesis: Is the Chemical Origin of Life a Realistic Scenario?
- Evolution Deception in California State High School Biology Textbook Biology: Principles & Explorations
- Cell Membrane-Like Organic Vesicles Formed in Conditions Mimicking Interstellar Clouds?
- NASA Scientist Discovers Alien Life in Meteorites - Again! NOT!
- What's Wrong With NASA's Arsenic-Eating Bacteria Study?
- Origin of life: latest theories/problems
- The Origin of Life on Planet Earth
- Book Review: Origins of Life: Biblical and Evolutionary Models Face Off
Related Materials 
 Origins of Life:
Biblical and Evolutionary Models Face Off
Origins of Life:
Biblical and Evolutionary Models Face Off![]() by Fazale Rana and Hugh Ross. Probably the single most potent
scientific argument against atheism is the problem with a naturalistic origin of
life. This very problem led me to become a deist as a biology major at USC in
the early 1970's. The problems for atheists have gotten no better since that
time. In fact, the last 30+ years of research have turned up even more problems
than those that existed when I first studied the theories. Fuz Rana (a
biochemist) and Hugh Ross (an astrophysicist) have teamed up to write the
definitive up-to-date analysis of the origin of life. The book examines the origins of life from the
perspectives of chemistry, biochemistry, astronomy, and the Bible. A biblical
creation model is presented along side the naturalistic models to help the
reader decide which one fits the data better. This is an excellent book to give
to your unbelieving friends, since it presents a testable creation model that is
clearly superior to any naturalistic model.
by Fazale Rana and Hugh Ross. Probably the single most potent
scientific argument against atheism is the problem with a naturalistic origin of
life. This very problem led me to become a deist as a biology major at USC in
the early 1970's. The problems for atheists have gotten no better since that
time. In fact, the last 30+ years of research have turned up even more problems
than those that existed when I first studied the theories. Fuz Rana (a
biochemist) and Hugh Ross (an astrophysicist) have teamed up to write the
definitive up-to-date analysis of the origin of life. The book examines the origins of life from the
perspectives of chemistry, biochemistry, astronomy, and the Bible. A biblical
creation model is presented along side the naturalistic models to help the
reader decide which one fits the data better. This is an excellent book to give
to your unbelieving friends, since it presents a testable creation model that is
clearly superior to any naturalistic model.
References 
- Cohen, J. 1995. Getting All Turned Around Over The Origins of Life on Earth. Science 267:1265-1266.
- Cronin, J. R. and S. Pizzarello. 1997. Enantiomeric Excesses in Meteoritic Amino Acids. Science 275: 951-955.
- Pizzarello, A., Y. Huang, and M. R. Alexandre. 2008. Molecular asymmetry in extraterrestrial chemistry: Insights from a pristine meteorite. Proc. Natl. Acad. Sci. U.S.A. 105: 3700-3704.
- Herd, C. D. K., et al. 2011. Origin and Evolution of Prebiotic Organic Matter As Inferred from the Tagish Lake Meteorite. Science 332: 1304-1307.
- J. Martyn Bailey. 1998. RNA-directed amino acid homochirality. The FASEB Journal 12: 503-507.
- Tamura, K. and P. R. Schimmel. 2006.
Chiral-selective aminoacylation of an RNA minihelix: Mechanistic features
and chiral suppression.
PNAS 103: 13750-13752.
Tamura, K. 2008. Mechanism of chiral-selective tRNA aminoacylation and the origin of amino acid homochirality. Nucleic Acids Symposium Series 52: 415-416. - Hussain, T., S. P. Kruparani, B. Pal, A. Dock-Bregeon, S. Dwivedi, M. R. Shekar, K. Sureshbabu and R. Sankaranarayanan. 2006. Post-transfer editing mechanism of a D-aminoacyl-tRNA deacylase-like domain in threonyl-tRNA synthetase from Archaea. The EMBO Journal 25: 4152-4162.
- Klussmann, M., H. Iwamura, S. P. Mathew, D. H. Wells Jr., U. Pandya, A. Armstrong, and D. G. Blackmond. 2006. Thermodynamic control of asymmetric amplification in amino acid catalysis. Nature 441: 621-623.
- Lahav, N., D. White, and S. Chang. 1978. Science 201:
607.
Orgel, L. E. 1989. J. Mol. Evol. 29: 465.
Brack, A. 1993. Pure Appl. Chem. 65: 1103. - Huber, C. and G. Wächtershäuser. 1998. Peptides by Activation of Amino Acids with CO on (Ni,Fe)S Surfaces: Implications for the Origin of Life. Science 281: 670-672.
- Pizzarello, S. and A. L. Weber. 2004. Prebiotic Amino Acids as Asymmetric Catalysts. Science 303: 1151.
- Engel, M. H. and S. A. Macko. 1997. Isotopic evidence for extraterrestrial non- racemic amino acids in the Murchison meteorite. Nature 389: 265-268.
- Mason, Stephen F. 1997. Extraterrestrial handedness. Nature 389: 804.
- Bailey, J. 1999. Polarized Stellar Light. Science 283: 1415.
Bailey, J., A. Chrysostomou, J. H. Hough, T. M. Gledhill, A. McCall, S. Clark, F. Menard, and M. Tamura. 1998. Circular Polarization in Star- Formation Regions: Implications for Biomolecular Homochirality. Science 281: 672-674. - Kahr, B., and J.H. Freudenthal. 2008. Dendritic crystal growth, differential circular scattering, and the origin of biomolecular homochirality. Chirality 20: 973-7.
http://www.godandscience.org/evolution/origin_homochirality.html
Last Modified June 15, 2011